Ultrahuman, a leader in wearable health technology, announced in January 2026 a partnership with Click Therapeutics to develop the Migraine PowerPlug. This new software feature within the Ultrahuman platform aims to deliver a personalised, biomarker-driven migraine management tool.
According to Ultrahuman's official announcement, it leverages the proprietary technology behind Click Therapeutics’ FDA-authorised prescription digital therapeutic, CT-132, offering both insights and guided activities for users.
A New Approach to Migraine Management
The Migraine PowerPlug targets the over one billion people globally affected by migraine, a condition that impacts women nearly three times more than men. Migraine is a chronic neurological condition and a leading cause of disability among women aged 15–49, significantly affecting daily life and overall health.
Ultrahuman has focused on women's health in its roadmap, and this new feature aims to help users understand how hormonal fluctuations and other biometric shifts may relate to migraine risk, frequency, and severity.
Mohit Kumar, CEO of Ultrahuman, said, “Helping people improve how they feel daily is core to Ultrahuman’s mission. By integrating the underlying principles of Click Therapeutics’ digital migraine treatment technology into our latest PowerPlug, we are enabling users to track and understand migraine-related patterns in real time, and then go further to turn those insights into actionable guidance.”
How the PowerPlug Works: Biomarkers and Digital Therapeutic Principles
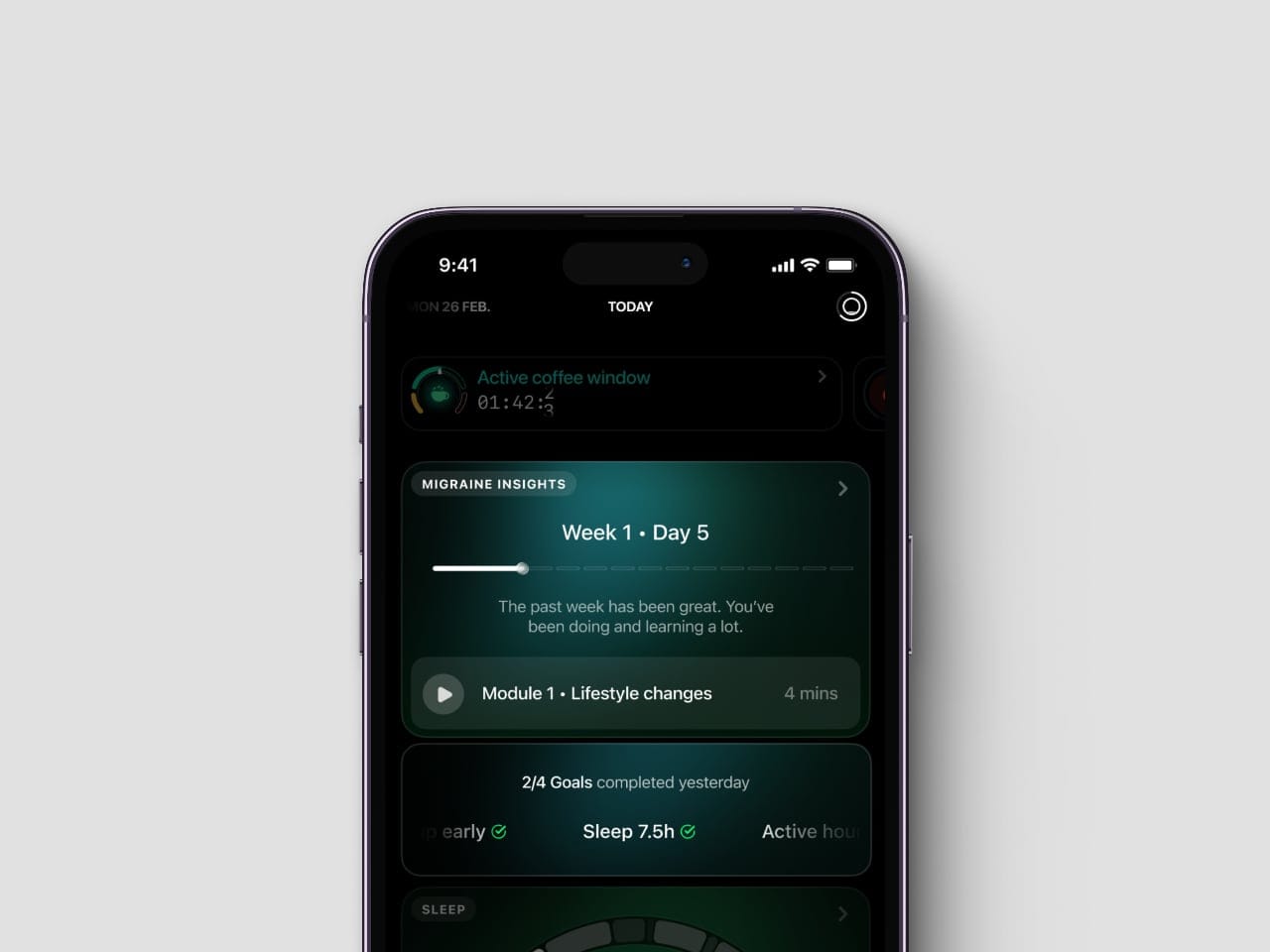
Current migraine management often relies on occasional clinical visits, despite symptoms and triggers occurring daily. The Ultrahuman Migraine PowerPlug is designed to extend care beyond the clinic, using wearable data and evidence-based interventions to support users in real-time. It combines Ultrahuman’s biomarker analytics with the technology and intellectual property behind Click Therapeutics’ CT-132, an FDA-authorised digital treatment for the preventive management of migraine.
Unlike general wellness tools that offer only tracking, the Migraine PowerPlug is developed using digital therapeutic principles. This helps users understand how biometric changes across sleep, recovery, stress, and activity may correlate with potential triggers. It then translates these trends into practical, personalised lifestyle guidance, enabling users to spot patterns and build consistency to improve resilience over time. For more on how wearables can help with health, check out our guide on wearable health technology.
Bridging the Gap in Wearable Health
This partnership marks a significant step in the digital health landscape. David Benshoof Klein, CEO and founder of Click Therapeutics, noted, “Our partnership with Ultrahuman will allow us to bring a much-needed personalised migraine solution to people around the world. As digital health evolves, combining our clinically validated digital treatments with personalised consumer biometrics allows us to address an unmet need in over-the-counter care and the fast-growing wearables market.”
It's important to remember the Migraine PowerPlug is a general wellness and educational experience. It is not a prescription digital therapeutic and is not intended to diagnose, treat, cure, or prevent any disease, nor does it replace professional medical care. Ultrahuman continues to expand its health ecosystem, including products like the Ultrahuman M1 CGM, empowering people to act early and live better.
Migraine PowerPlug Availability
The Migraine PowerPlug is scheduled to launch for users via the Ultrahuman app in early 2026, following a pilot phase. Initial regions for availability include the United States, Canada, the EU, India, Australia, and more. Specific launch dates, pricing, or explicit confirmation for the UAE market have not been announced. Availability and features may vary by region.
Frequently Asked Questions
What is the Migraine PowerPlug?
The Migraine PowerPlug is a software-based feature within the Ultrahuman platform, developed in partnership with Click Therapeutics. It is designed to offer biomarker-driven migraine-related insights and guided activities, leveraging principles from FDA-authorised technology to help users better understand and manage their migraine patterns.
How does Migraine PowerPlug work?
It combines Ultrahuman’s biomarker analytics, such as sleep, heart rate variability (HRV), stress, and activity, with the technology behind Click Therapeutics’ FDA-authorized digital therapeutic (CT-132). This helps users understand how biometric changes correlate with potential migraine triggers and provides guided behavioral interventions for proactive management.
When will Migraine PowerPlug be available?
The Migraine PowerPlug is scheduled to launch for users via the Ultrahuman app in early 2026, after a pilot phase. Regions for its initial rollout include the US, Canada, EU, India, Australia, and potentially others. Specific UAE availability and a precise launch date are yet to be confirmed.
Is Migraine PowerPlug an FDA-approved treatment?
The Migraine PowerPlug is based on the proprietary technology behind Click Therapeutics’ FDA-authorized prescription digital therapeutic (CT-132). However, the PowerPlug itself is described as a general wellness and educational experience. It is not a prescription digital therapeutic and is not intended to diagnose, treat, cure, or prevent any disease.
Subscribe to our newsletter to get the latest updates and news













Member discussion